Background
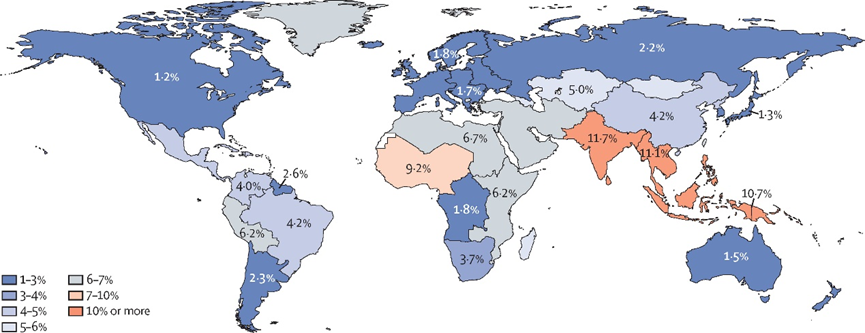
"The eye is the window to the soul." This famous quote by Leonardo da Vinci precisely highlights the critical role of vision in how humans perceive the world. However, cataracts are the most common cause of blindness globally[1], affecting tens of millions of people annually, especially among middle-aged and elderly populations.
Currently, the mainstream treatment for cataracts involves surgically removing the cloudy lens and implanting an artificial intraocular lens. This method demands substantial medical resources and is not widely accessible in underdeveloped regions. According to the American Academy of Ophthalmology, surgery remains the gold standard for cataract treatment[2].
Existing pharmacological treatments, such as eye drops, face challenges like poor drug absorption, short retention time, and the burden of frequent administration, making it difficult to achieve ideal therapeutic outcomes[3]. For example, antioxidants and protein aggregation reversal agents have shown some efficacy in cataract models, but due to the anatomical and physiological barriers of the eye, traditional eye drops face limited effectiveness[4]. Although nanotechnology has made some progress in drug delivery, further research is needed to solidify the therapeutic efficacy of these compounds and improve delivery methods[5].
Core Technology
1. Molecular Mechanisms of Cataracts and Treatment Bottlenecks
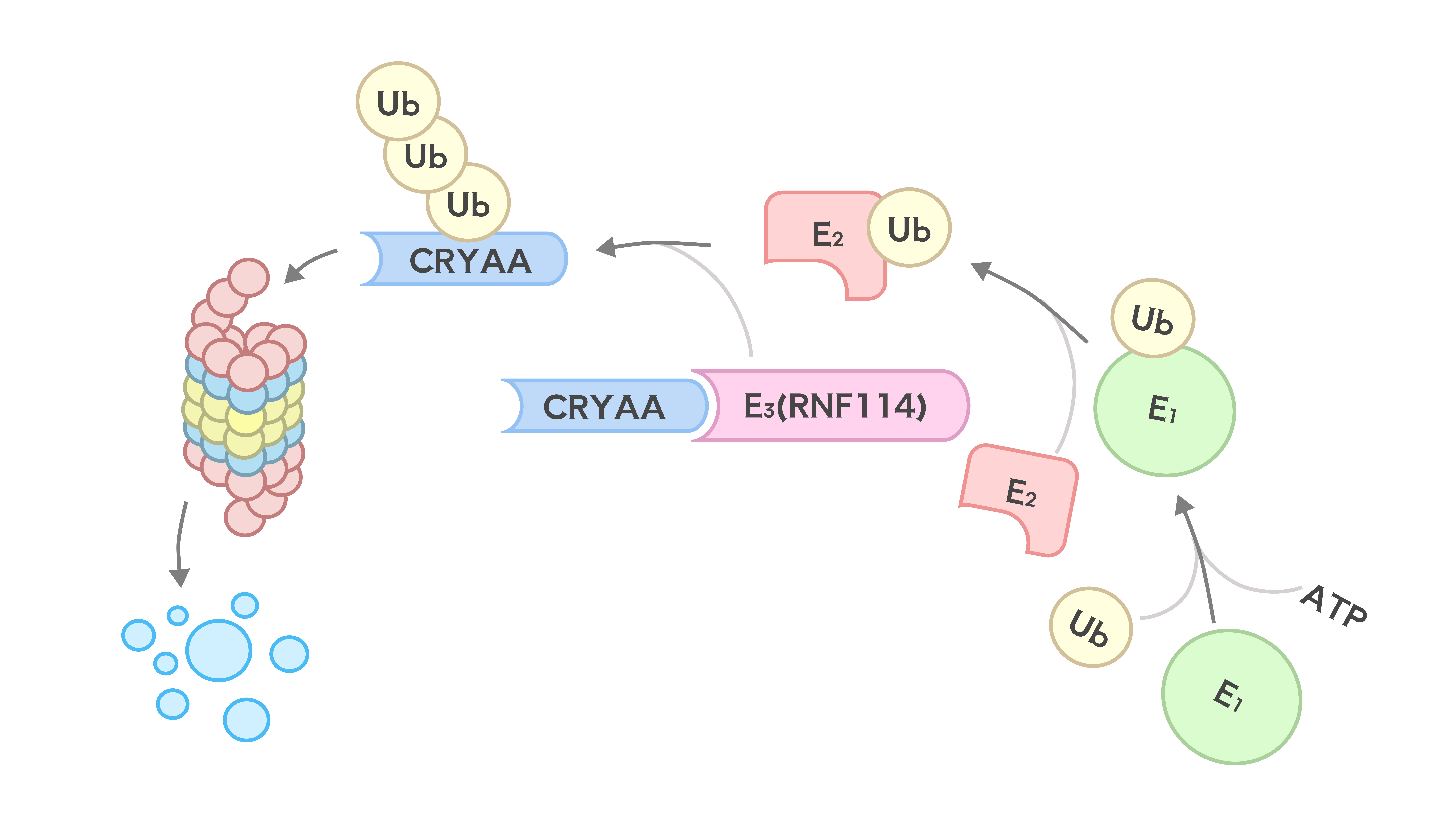
The core pathology of cataract formation is linked to the misfolding and aggregation of lens proteins, with the small heat shock protein CRYAA playing a crucial role. When its function is impaired, environmental stressors (such as low temperature and oxidative stress) or genetic mutations (like the CRYAA Y118D mutation) can cause CRYAA to lose its chaperone activity. This accelerates protein aggregation and the formation of insoluble deposits. Such protein aggregates lead to lens opacity, resulting in visual impairment or even blindness[6][7][8][9].
To break this vicious cycle, the team selected the E3 ubiquitin ligase RNF114. RNF114 precisely identifies misfolded CRYAA proteins and tags them for degradation through ubiquitination, thereby restoring lens transparency. Research has shown that RNF114 significantly reduces lens opacity in cold-induced and oxidative stress-related cataract models[10]. This therapeutic target offers unprecedented breakthrough potential.
2. Innovative Solution: Contact Lens-Based Drug Delivery System
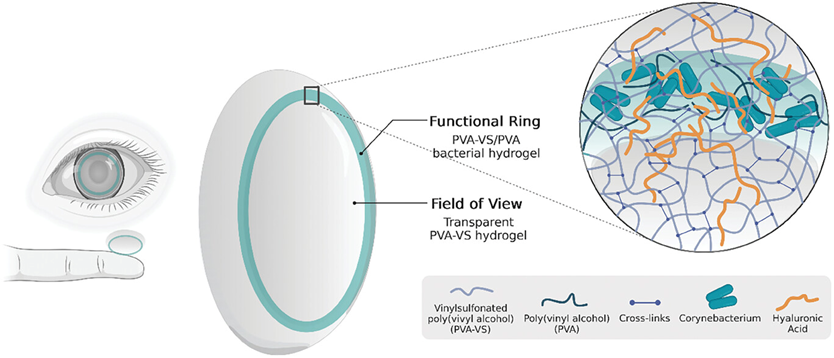
Our team proposes a novel treatment approach: using hydrogel-based contact lenses as drug delivery carriers. Through bioengineering and modern materials science, we've designed contact lenses capable of sustained drug release. By modifying the biohydrogel system originally used for secreting hyaluronic acid to treat dry eye[11], we engineered it to simultaneously secrete RNF114 for cataract treatment[10].
This not only enables continuous drug release but also targets the lens, fundamentally addressing the limitations of existing drug therapies. It reduces the burden of frequent drug administration for patients, aims to maintain higher average drug concentrations, and lowers systemic toxicity.
Key Technological Modules Include:
-
Biohydrogel Platform: Utilizing biohydrogel as the base material for contact lenses, we've embedded micro "biofactories" at the peripheral edges without affecting the visual field. These biofactories, engineered through synthetic biology, can continuously secrete therapeutic agents like RNF114 protein and hyaluronic acid, ensuring long-term drug delivery.

-
Dual Treatment for Dry Eye: The secretion of hyaluronic acid not only lubricates the eye surface, alleviating dry eye caused by prolonged contact lens wear, but also creates a stable environment for the release of other drug components. Additionally, hyaluronic acid acts as an excipient to enhance drug stability.
-
Targeted Delivery Capability: Through molecular modifications, the drug is engineered to penetrate the cornea and target the lens specifically, ensuring both precise delivery and stability.
3. Advantages of Contact Lenses in Drug Delivery
Compared to traditional eye drops, contact lenses offer significant technological advantages:
-
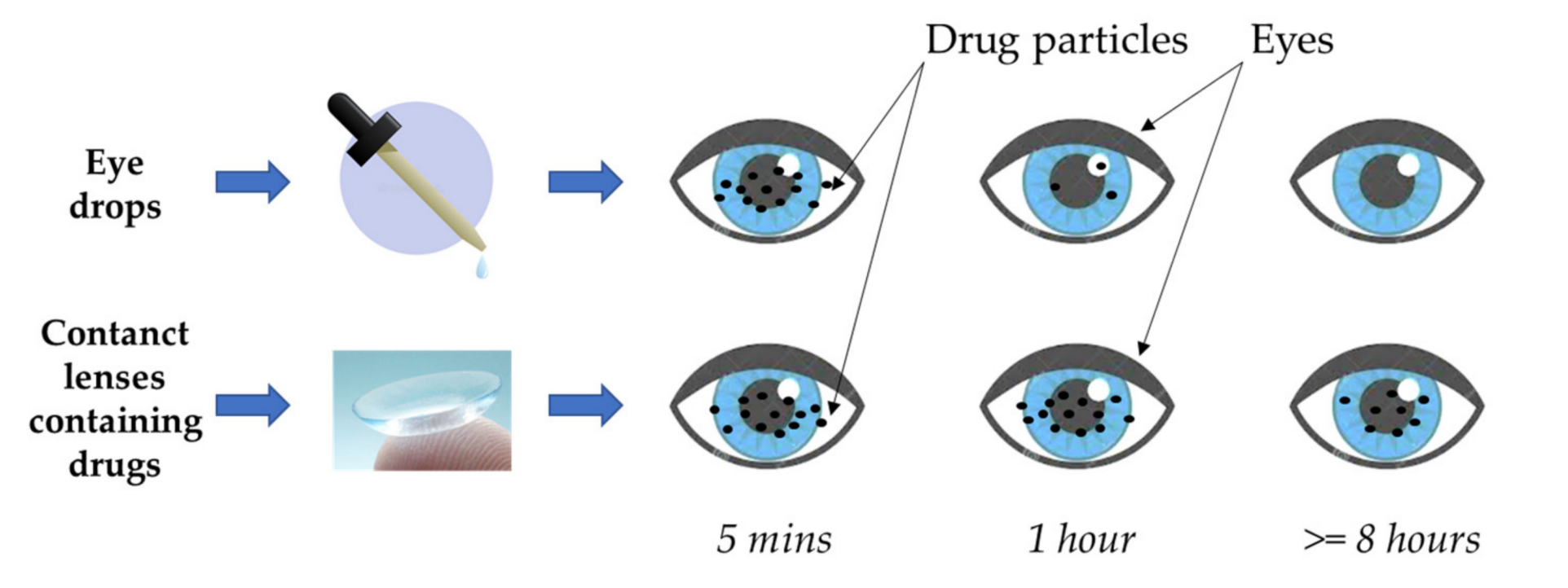
Prolonged Drug Retention Time: Approximately 95% of the active substances in conventional eye drops are lost through tear drainage[12]. Contact lenses, however, fit closely to the eye surface, reducing drug loss due to blinking or tear flow and significantly extending drug retention time on the cornea. This design greatly improves drug bioavailability, fundamentally addressing the low absorption rates of eye drops.
-
Sustained Drug Release Mechanism: Using the selective permeability of hydrogels, the team fixed engineered bacteria to continuously produce and release active drugs. The microporous structure of the hydrogel further extends drug release duration, maintains stable drug concentrations in target tissues, and reduces the need for frequent administration.
4. Drug Molecule Design and Optimization
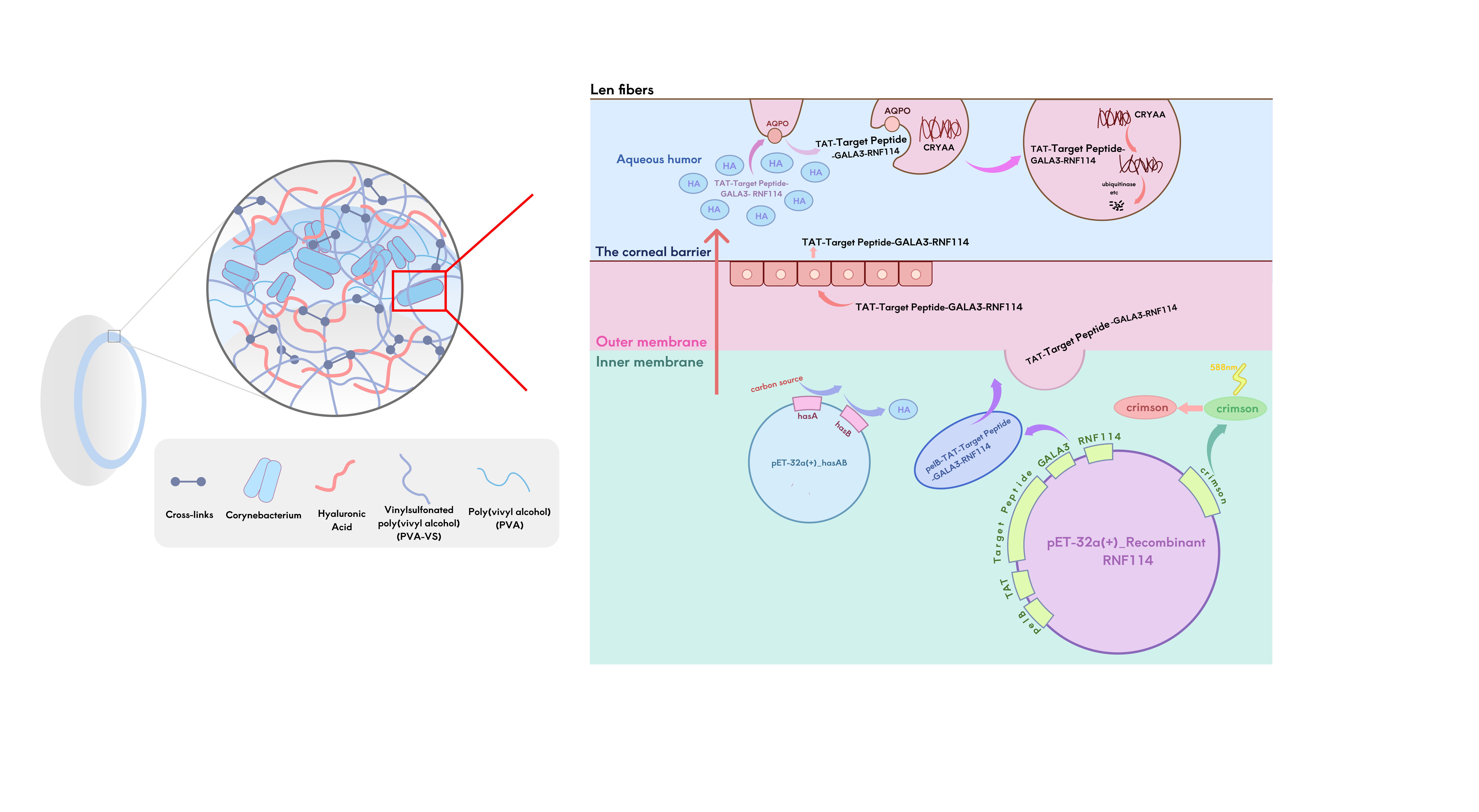
We have identified a non-invasive, contact lens-based biological factory designed for the sustained release of therapeutic agents to treat cataracts. Our primary therapeutic component is RNF114, expressed using the SHuffle E. coli system as the chassis. To achieve this, we introduced an exogenous gene to enable successful RNF114 expression within SHuffle.
We obtained the human RNF114 sequence from NCBI. Since prokaryotic cells lack the splicing mechanisms found in eukaryotic cells, we aimed to extract the CDS (coding sequence) via PCR, eliminating introns to overcome the absence of post-transcriptional splicing.
However, the efficacy of non-invasive drug delivery systems within the eye is severely hindered by natural ocular defense barriers [13]—including the antimicrobial clearance mechanisms of the tear film, multiple corneal barriers, and aqueous humor dilution and drainage, all of which interfere with drug delivery. Conventional methods like liposomal encapsulation are unsuitable for continuous drug release within engineered biological systems.
To address these challenges, we employed protein modification techniques to enhance drug delivery efficiency through improvements in permeability, stability, and targeting capability.
Enhancing Permeability
-
Signal Peptide for Secretion: Target proteins synthesized by engineered bacteria require guidance for successful secretion. We selected pelB as the signal peptide for directing the secretion of RNF114.The PelB signal peptide is derived from the amino-terminal leader sequence of PelB of Erwinia carotovora. It is a heterologous signal peptide currently widely used in the Escherichia coli expression system and has a wide range of applications in the secretion and expression of human recombinant therapeutic proteins.
-
TAT Peptide Modification: TAT-(47–58), a cell-penetrating peptide (CPP) derived from the HIV-1 regulatory protein TAT, enhances membrane permeability through various mechanisms. It shows great potential in facilitating drug delivery into target cells[14], with proven applications across multiple systems, including the eye. Studies have shown that coupling TAT peptides with RNF114 significantly improves lens clarity in cataract models. We plan to modify RNF114 with TAT peptides to boost its transmembrane transport while incorporating flexible linkers to preserve the protein's 3D structure, enabling efficient corneal penetration.
-
Endosomal Escape Mechanism (GALA Peptide): Although CPPs like TAT enhance the uptake of bioactive macromolecules via endocytosis, many internalized proteins become trapped within endosomes and are eventually degraded in lysosomes. To counteract this, we identified the GALA peptide, a synthetic amphiphilic peptide derived from mutated sequences of the HA2 protein. GALA exhibits strong membrane-disruptive properties when used in cationic liposomes and nanocarriers for drug or nucleic acid delivery. Notably, a redesigned version, GALA3, significantly improves endosomal escape efficiency for fusion proteins like BLF1[15]. We plan to attach GALA peptides to RNF114, enhancing endosomal escape for optimal therapeutic efficacy.
Improving Stability
To address RNF114 protein degradation due to ocular surface tear fluid, we initially considered methods like PEGylation or glycosylation for stabilizing vulnerable sites. However, these chemical conjugation techniques are incompatible with our genetic circuit strategy. Directed evolution was explored but abandoned due to the lack of a method to link mutations to cellular survival. Protease inhibitors were also rejected for potentially disrupting normal cellular functions.
Ultimately, we focused on Polyol Co-Release for Protein Stabilization and Rare Amino Acid Substitution to enhance protein stability by stabilizing its conformation and shielding critical sites from degradation.
Polyol Co-Release for Protein Stabilization
Polyols stabilize proteins by enhancing hydration. By forming a protective hydration shell that reduces enzyme access to cleavage sites, polyols lower the degradation rates of proteins. They also prevent protein aggregation, preserving proteins in monomeric or properly folded forms, which further decreases proteolytic degradation [16].
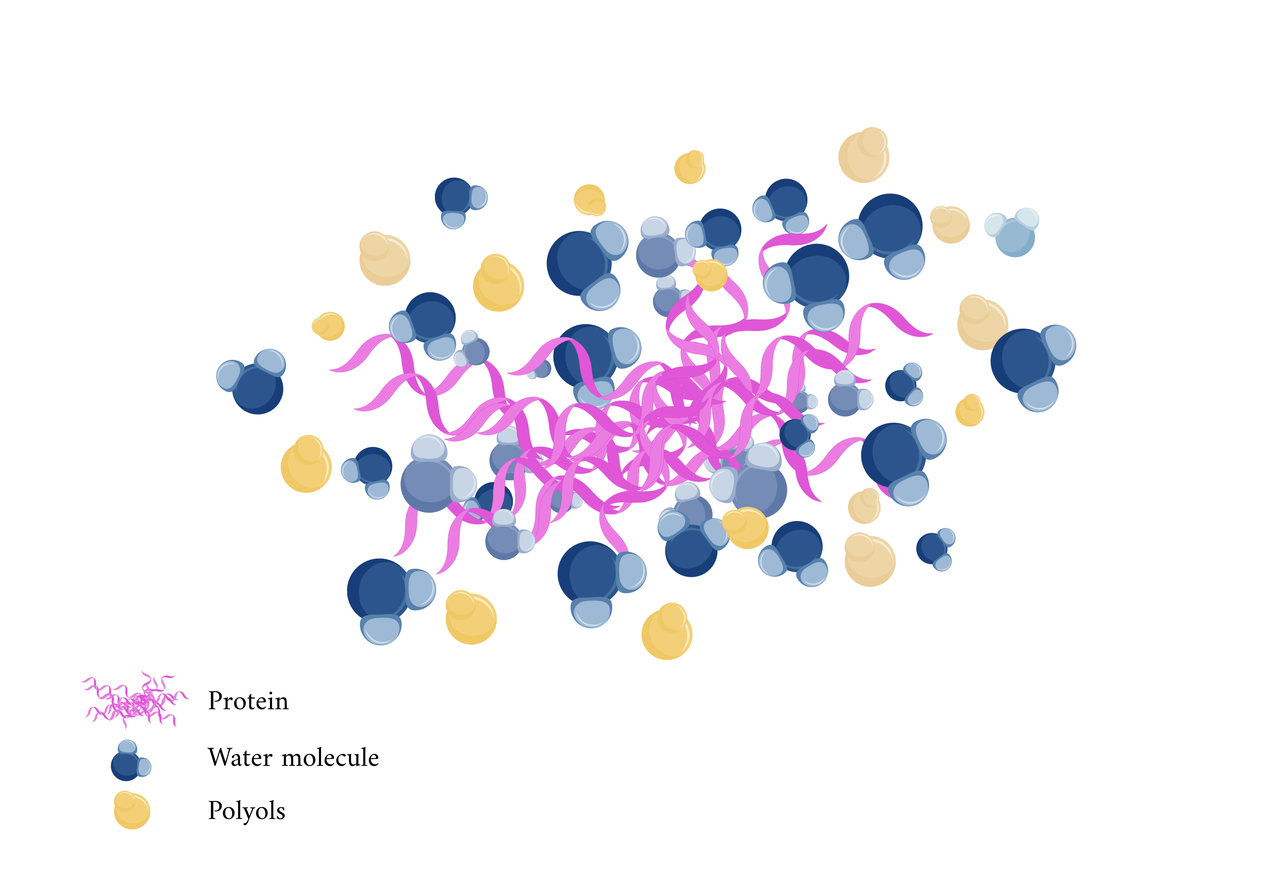
Interestingly, we discovered that the hyaluronic acid (HA) secretion module, initially designed to alleviate contact lens-induced dry eye, offers unexpected benefits. In addition to its moisturizing effects, HA serves as a promising ocular drug delivery excipient. Its high biocompatibility, biodegradability, and low immunogenicity enhance drug bioavailability. Currently available studies include drugs that utilize hyaluronic acid to act on the corpus cavernosum, conjunctiva, and anterior segment of the eye. Among which, Pilocarpine acting on the pupillary sphincter.
We hypothesize that this drug protects its stability at the ocular surface to the aqueous humor. Thus, the stability of the drug molecule can be ensured by synchronized release of HA.
Rare Amino Acid Substitution (Backup Strategy)
If RNF114 remains unstable after being encapsulated by hyaluronic acid, the replacement of specific amino acids can be considered. Using ExPASy PeptideCutter to predict the sensitive sites of RNF114 degradation by proteases, statistics show that phenylalanine at positions 27, 99, 151, 191, and 206 are all cleaved by enzymes five times. Therefore, these five positions are selected as replacement sites, and their codons are modified to UAG.
The pEVOL-pAzf plasmid can generate a tRNA synthetase/tRNA pair that recognizes UAG and carries p-azidophenylalanine[17]. Introducing this plasmid into the strain can replace phenylalanine at the target positions with p-azidophenylalanine. The structures of these two amino acids are similar, so the replacement will not significantly affect the activity of RNF114, and the azido group can interfere with protease recognition.
Due to the complexity of this approach, it will serve as a contingency plan if HA-based stabilization proves insufficient.
Targeting Modifications
To ensure that the RNF114 protein is accurately delivered to the lens, we chose AQP0 as the targeting receptor. AQP0 is a water channel protein that is almost exclusively found in the lens fiber cell membrane of mammals, ensuring high specificity. It exists in both connected and non-connected forms[18] and can interact with various proteins[19]. Through software modeling, we designed a short peptide that can specifically bind to AQP0, enabling precise delivery.
Protein Design
Selecting the Attachment Site
Observing the dimensional structure of the drug to be modified, RNF114 (RNF114 3D structure), its possible site of action, the zinc finger, is located at amino acids 29-68 and 91-110 of the protein. We found that the N-terminal sequence of RNF114 is less folded, less confident, not a zinc finger, and less conserved, making it a suitable site for modification. Therefore, we chose to add the additional structure at the end of the N-terminus of RNF114. Meanwhile, at the experimental stage, in order to quickly monitor the expression of RNF114, we attached GFP (green fluorescent protein) to the C-terminal end of the RNF114 protein via multiple copy number of rigid linker + flexible linker.
Determining the Connection Sequence
-
Signal Peptide (pelB): Functions within engineered bacteria, guiding protein secretion.
-
TAT Peptide: Facilitates drug penetration through the corneal barrier and entry into target cells.
-
Targeting Peptide: Binds specifically to AQP0 on lens fiber cell membranes, ensuring accurate drug localization.
-
GALA Peptide: Responds to pH changes, aiding in endosomal escape within target cells to ensure the drug reaches its site of action.
Considering that pelB is cleaved during protein secretion, we positioned it at the N-terminal end of RNF114.
Since GALA peptides require conformational flexibility to function effectively, we added longer linkers to prevent structural interference with adjacent protein modules.
Linker Selection
We used alphafold3 to evaluate linkers of varying lengths—GS3, GS5, and GS9[20]—and assessed both flexible and rigid linkers[24] respectively and we also tried to place the GALA peptide at the c-terminal end to test whether it could reduce the structural alteration of the GALA peptide on the protein. Finally, we found that the protein fusion method in the figure below had the least effect on the drug structure.
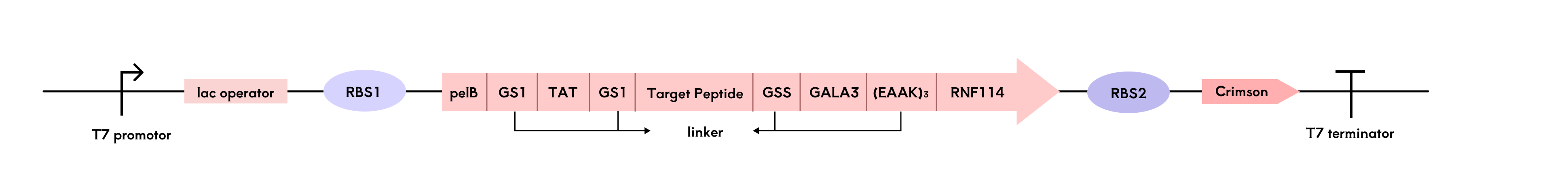
Ultimately, we identified the fusion protein configuration that preserved RNF114's structural integrity and functional activity most effectively.
5. Chassis Design and Production Method
In order to ensure the stable expression and production of the drug, we chose the SHuffle E. coli expression system as the biofactory. It not only has enhanced oxidative folding ability and provides a standard plasmid backbone. In the future, if needed, multiple expression frameworks can be introduced to co-express auxiliary proteins required for RNF114 synthesis.
6. Biosafety
Biosafety Reporting Module
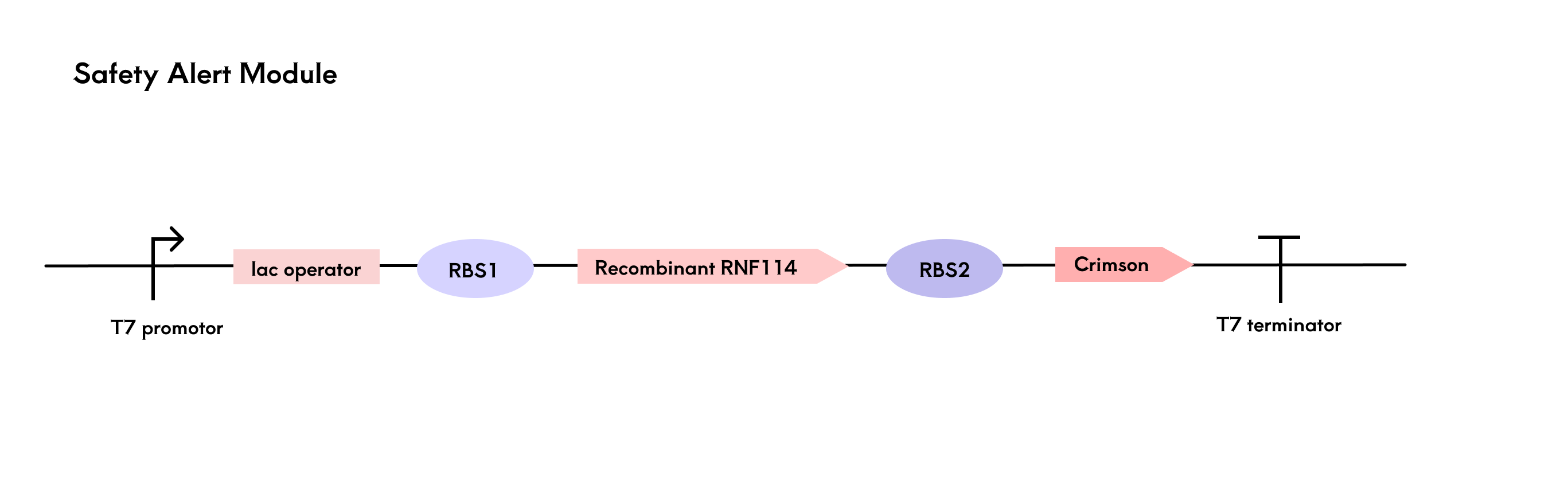
The engineered bacteria are encapsulated in a hydrogel and rely on nutrients from tear fluid for survival, influenced by factors such as temperature and nutrient elements in the ocular microenvironment. To provide timely alerts when bacterial activity declines or there is a risk of lysis, we designed a reporting module centered around the fluorescent protein Crimson[21].
Using a multi-cistronic structure, we co-expressed RNF114 and Crimson, adjusting the RBS strength to ensure higher expression of RNF114. After translation, both genes are located on the same mRNA. When bacterial activity decreases and RNF114 expression drops, Crimson expression also decreases, leading to a reduction in fluorescence signal and providing a safety risk report. As mentioned in the protein design module, GFP was used in the experimental phase to monitor RNF114 expression quickly. However, considering GFP's potential toxicity, only Crimson is retained in the final product design.
The fluorescence detection system is integrated into a black contact lens case. By pressing a button, the light source excites the Crimson fluorescence protein, and the BH1750 sensor detects the fluorescence intensity. Based on the threshold, the safety level is determined and displayed on an LCD screen.
Suicide Switch in case of Leakage
We have designed two suicide systems as strategies to prevent the leakage of engineered bacteria. In the auxotrophic bacterial system, we created two complementary nutrient-deficient SHuffle T7-B strains, where strain A is unable to synthesize tryptophan, and strain B is unable to synthesize histidine. These strains complement each other and provide the required amino acids through metabolic cooperation and overexpression mechanisms, ensuring they can grow when coexisting, but cannot survive individually due to the lack of essential nutrients.
In the phosphate-sensitive promoter killing switch, we control the expression of the suicide gene (mazF) using the phosphate-sensitive P_PhoB promoter, and use a hydrogel to gradually release phosphate into the environment of the engineered bacteria. This ensures that when phosphate is absent, the suicide mechanism is triggered, leading to bacterial death.
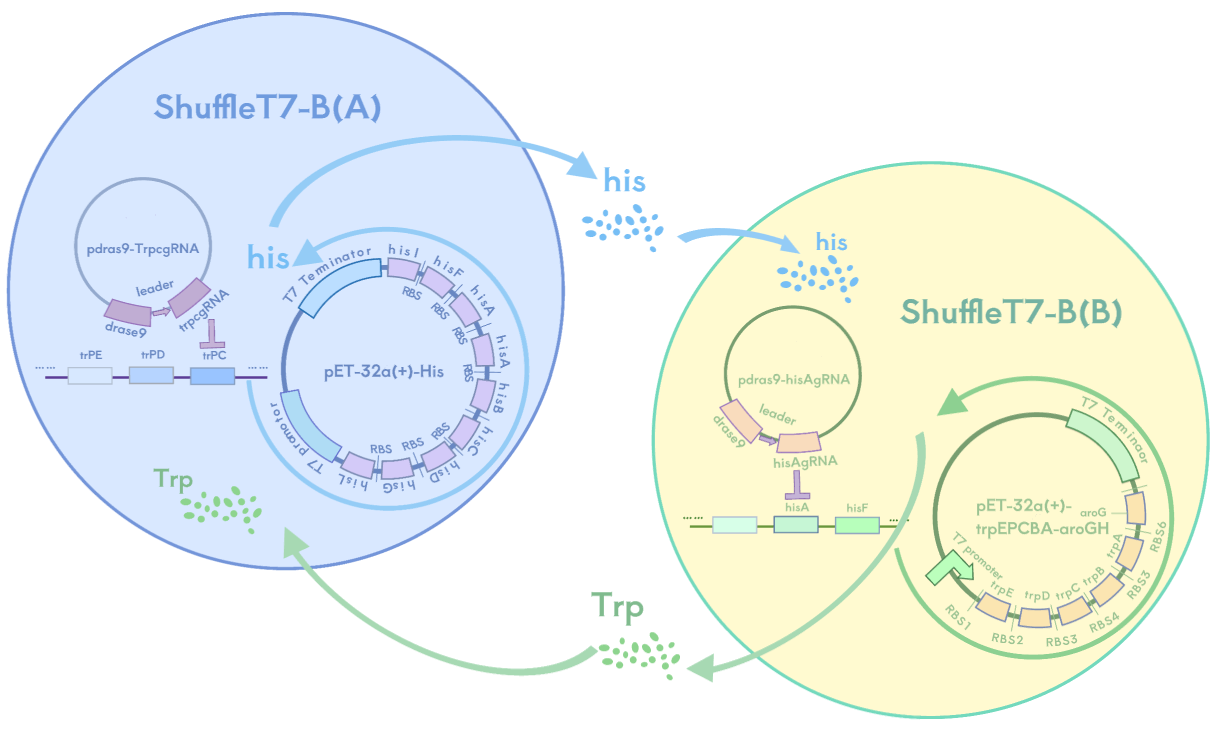
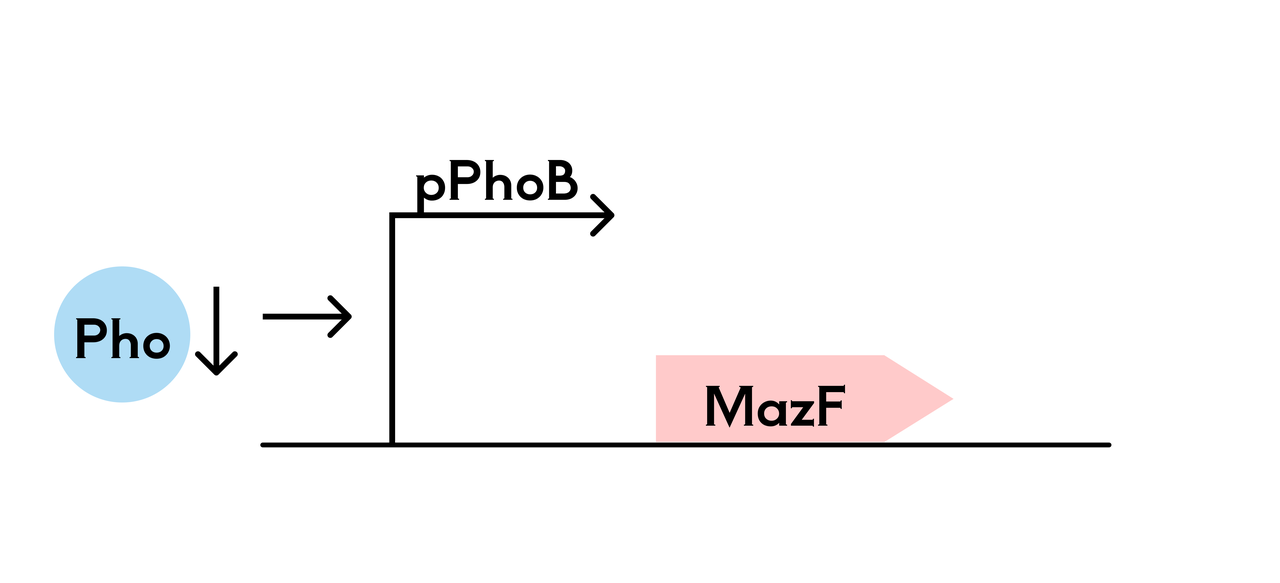
Both systems ensure the survival of the SHuffle T7-B strain in the hydrogel, while triggering the suicide mechanism in the external environment due to the lack of essential amino acids or phosphate, ensuring selective survival or death of the bacteria and preventing their spread to the eye or the natural environment.
7. Model Validation and Experimental Analysis
To verify the feasibility of our design, we conducted a series of multi-level experiments:
-
Molecular Modeling and Structural Prediction
We used AlphaFold to predict the 3D structure of RNF114 after modification with the TAT peptide. This step ensured that RNF114 retained its biological activity post-modification and that the structural changes introduced by TAT, GALA, and other linked peptides did not disrupt its therapeutic function.
-
Microengineered Biomimetic Ocular Models
We independently designed and constructed an in vitro eyeball simulation chip to deeply restore the physiological characteristics of tears, cornea, and aqueous reflux for evaluating the drug delivery effect of CLearCat. Compared with complicated animal experiments, this model also provides more options for the evaluation of ophthalmic drug delivery[22][23].
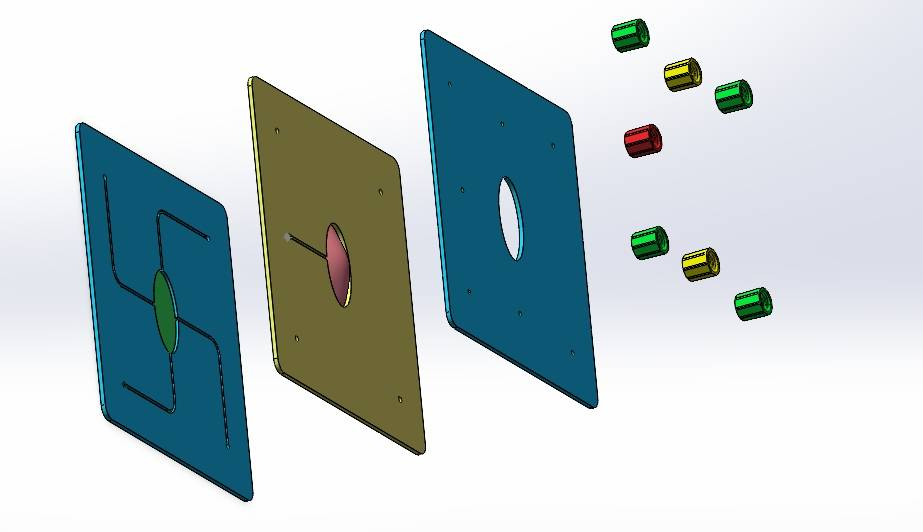
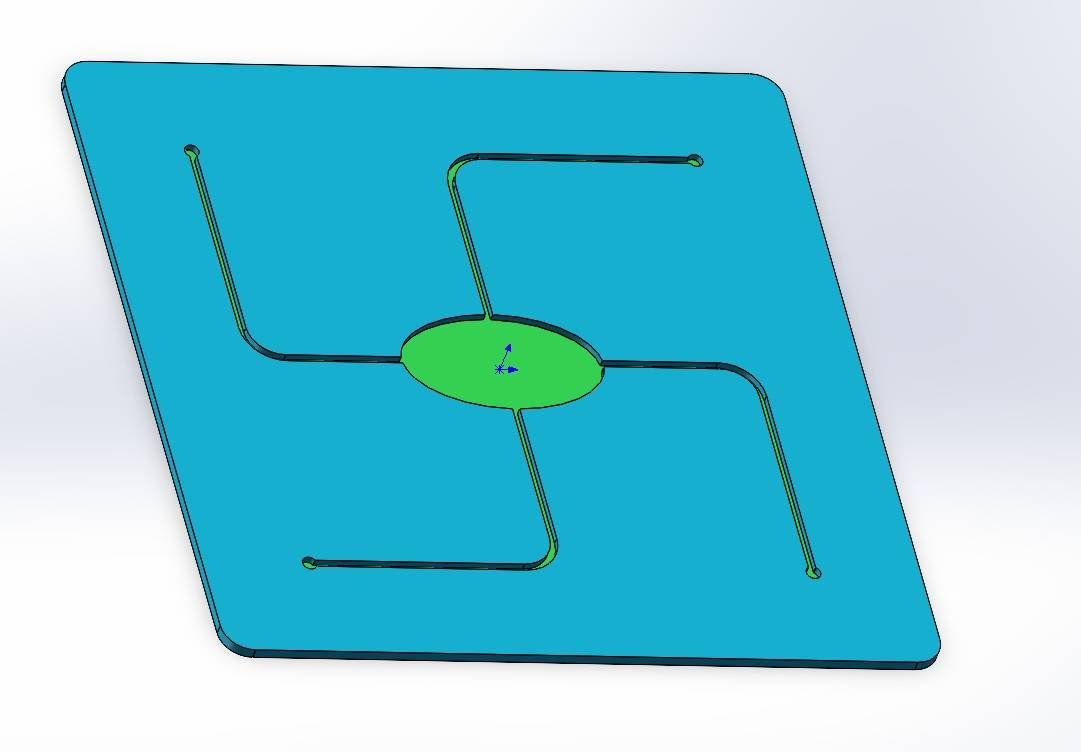
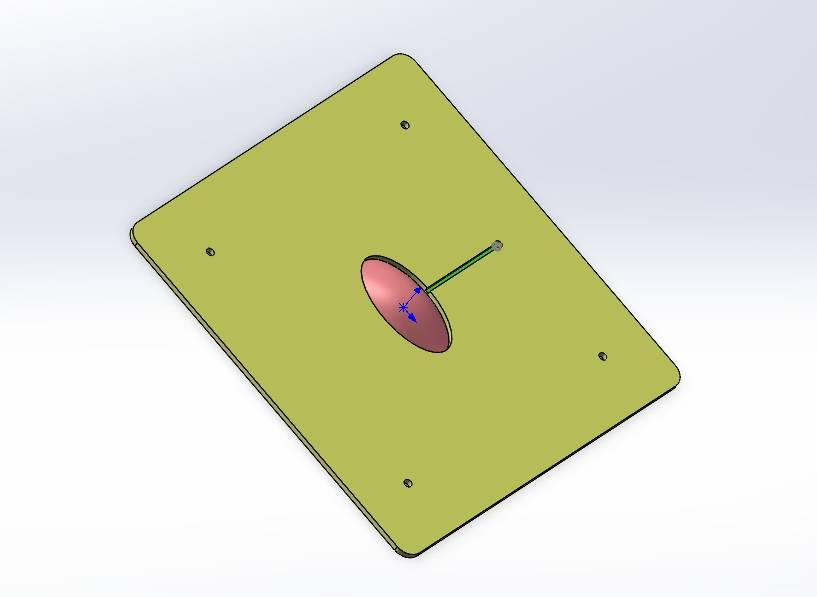
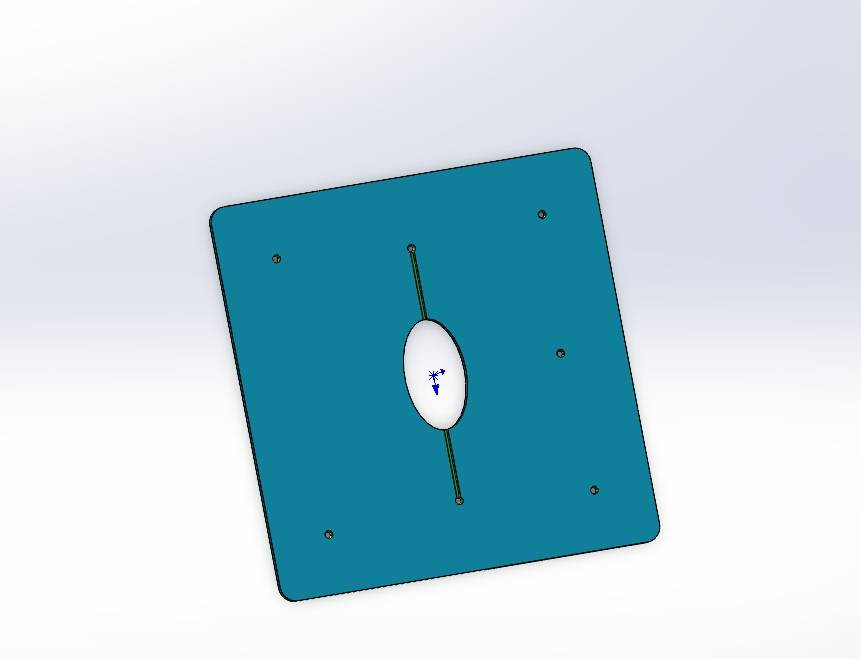
Figure 12: Microengineered biomimetic ocular model design showing different views: (1) Exploded view (2) Aqueous humor reflux (3) Cornea (4) Tear film
Prospects and Application Potential
The CLearCat drug delivery platform not only offers a breakthrough solution for cataract treatment but also opens new avenues for managing various other ophthalmic conditions.
-
Expanding to Other Eye Diseases: The core of this technology is highly scalable and can be expanded for the targeted treatment of glaucoma, retinal diseases, dry eye and other ocular diseases. As these diseases often require long-term treatment and localized drug delivery, our team has proposed a new solution to address this pain point: CLearCat. Compared with existing treatment options, CLearCat's long-acting drug release feature can significantly improve efficacy and reduce the burden of frequent drug administration, bringing a better therapeutic experience to patients.
-
Personalized Medicine Potential: The functional flexibility of CLearCat's contact lens platform makes it an ideal candidate for future integration with personalized medicine. Customizable based on individual genetic profiles or disease characteristics, CLearCat could one day provide tailor-made treatments for each patient, enhancing both efficacy and safety.
This marks a transformative shift in how we perceive contact lenses—from simple vision correction tools to sophisticated therapeutic devices. Such innovations not only expand the functional scope of contact lenses but also introduce entirely new treatment options for patients worldwide.
-
A Paradigm Shift in Ophthalmic Care: By merging modern biotechnology with materials science, CLearCat represents more than just a technical advancement—it's a potential revolution in ophthalmic medicine. This innovative platform challenges traditional treatment paradigms, offering hope to millions of patients suffering from visual impairments.
"Medicine is a science of uncertainty and an art of probability."
— William Osler
We believe that CLearCat's introduction will not only improve the quality of life for cataract patients but also bring new light and hope to millions globally.

