This project aims to provide breakthrough treatment solutions for eye diseases such as cataracts, with a focus on a long-acting drug delivery platform based on hydrogel contact lenses. The platform utilizes bioengineered miniature bioreactors to continuously secrete therapeutic components, such as RNF114. By adhering to the surface of the eye, the contact lenses overcome the issues of low drug absorption rates and short retention times associated with traditional eye drops, fundamentally improving the effectiveness of drug treatment while reducing systemic toxicity and the burden of frequent administration.
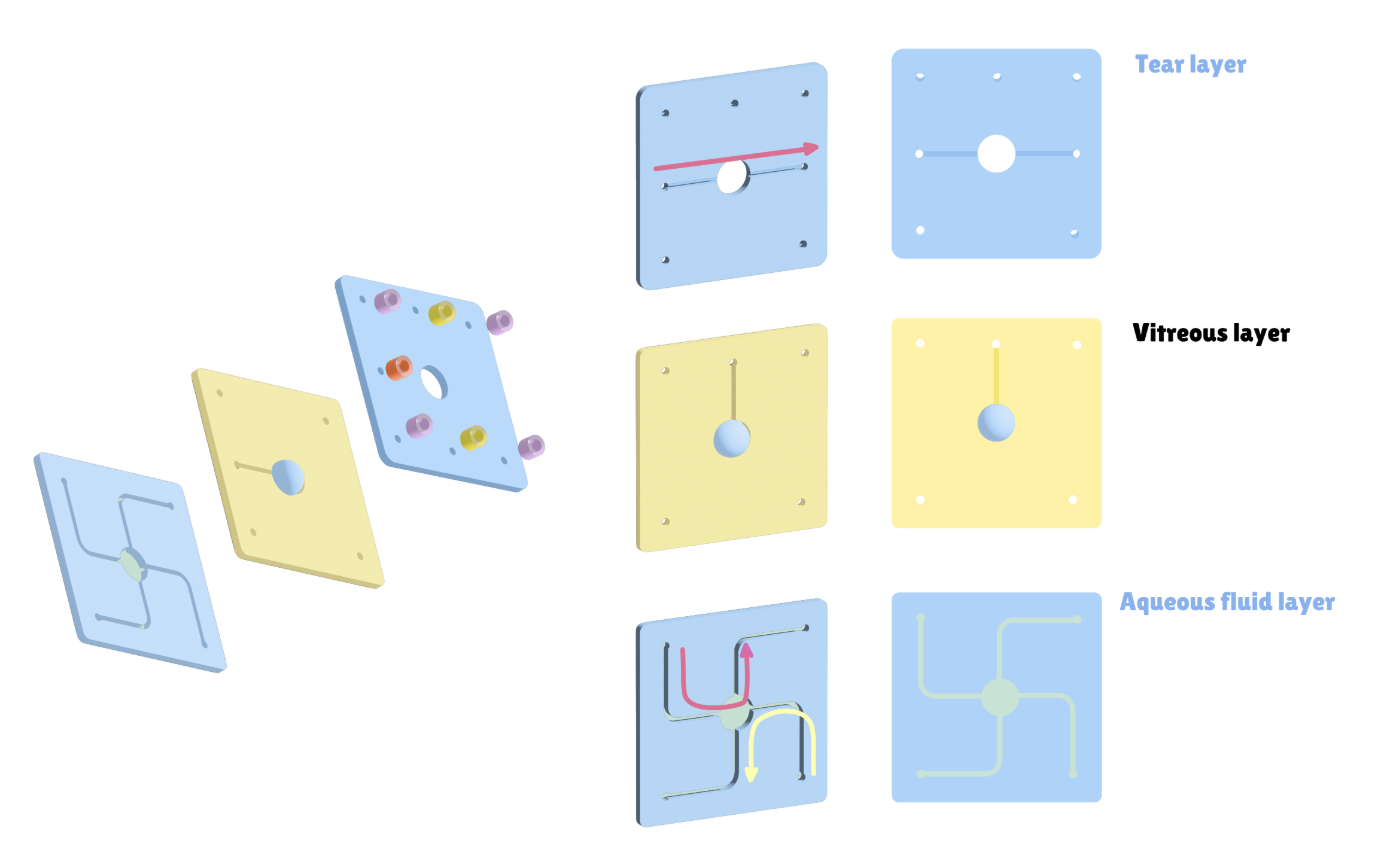
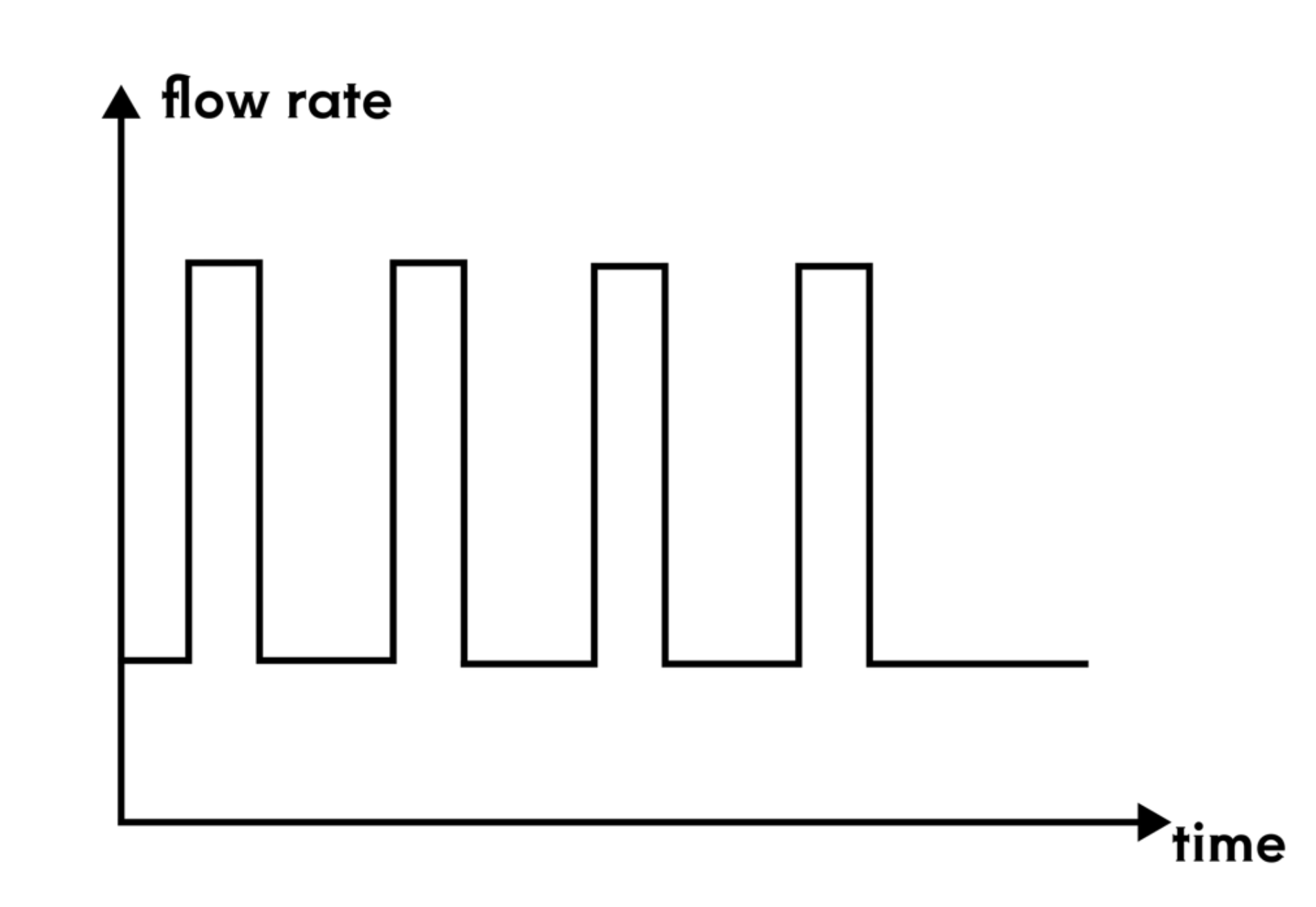
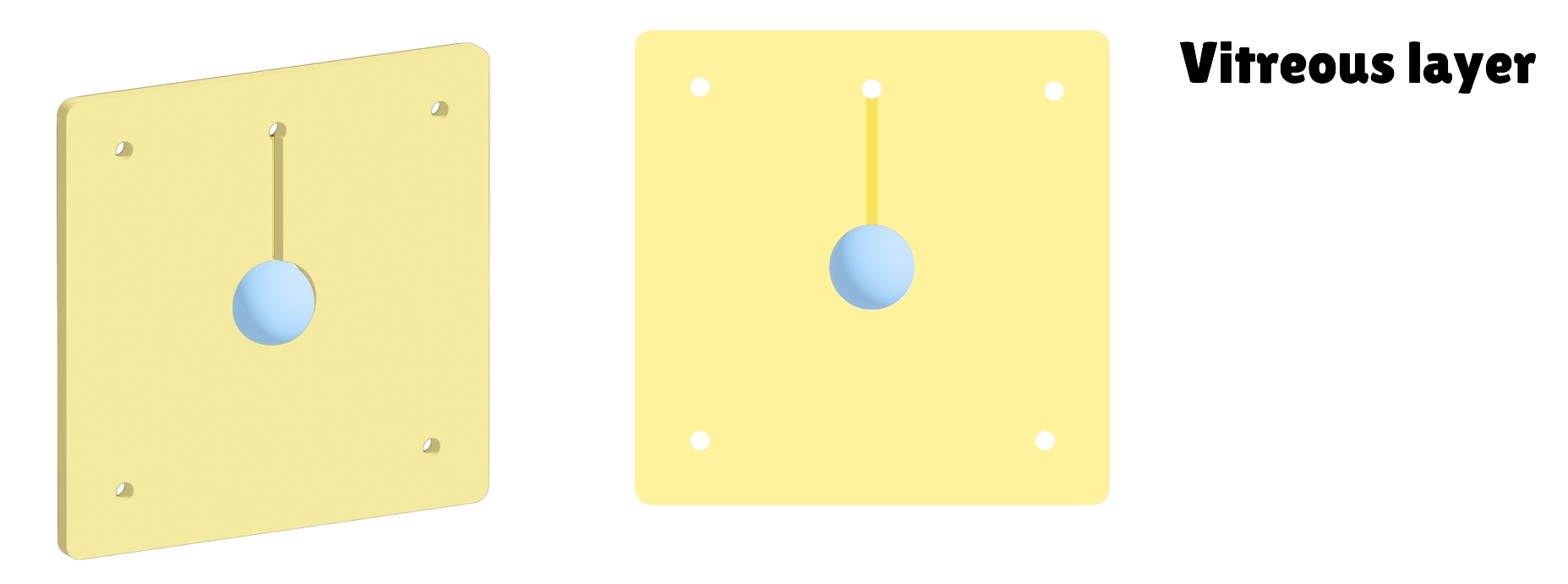
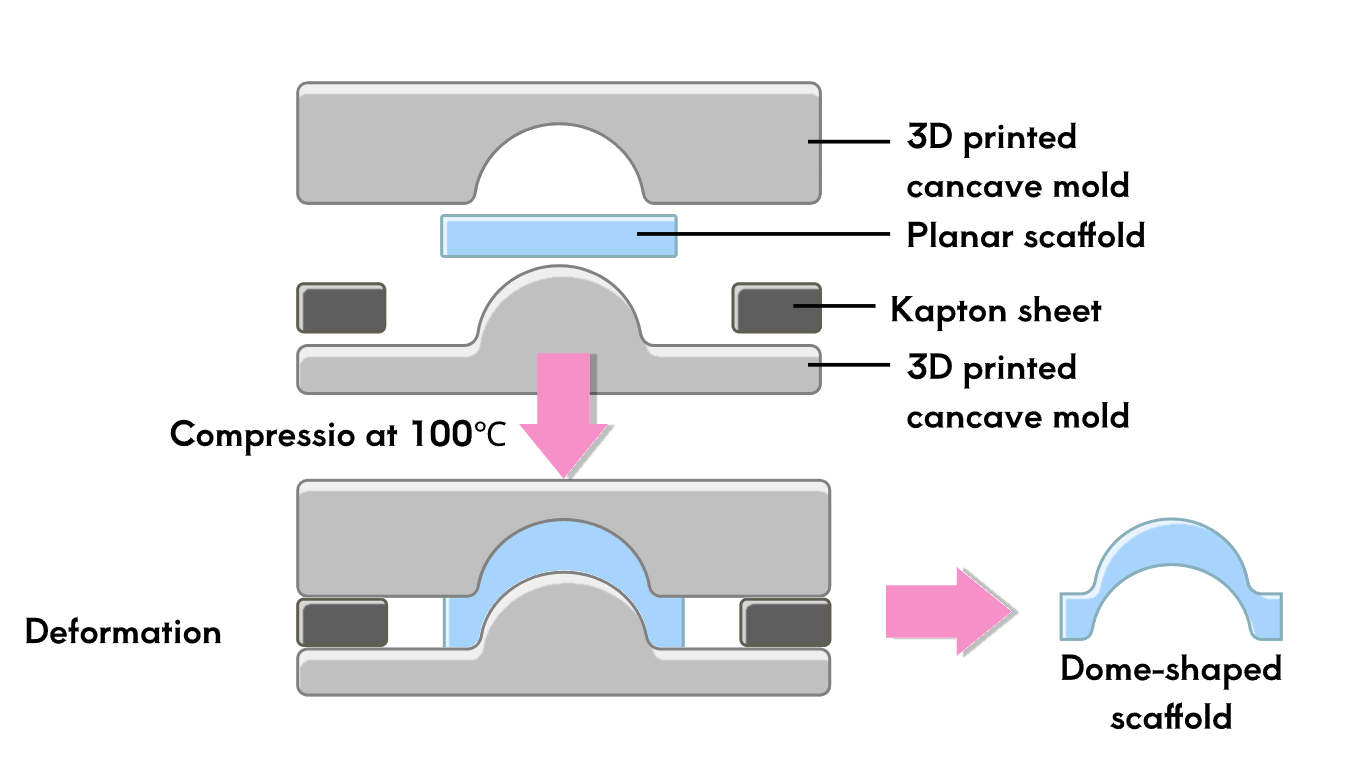
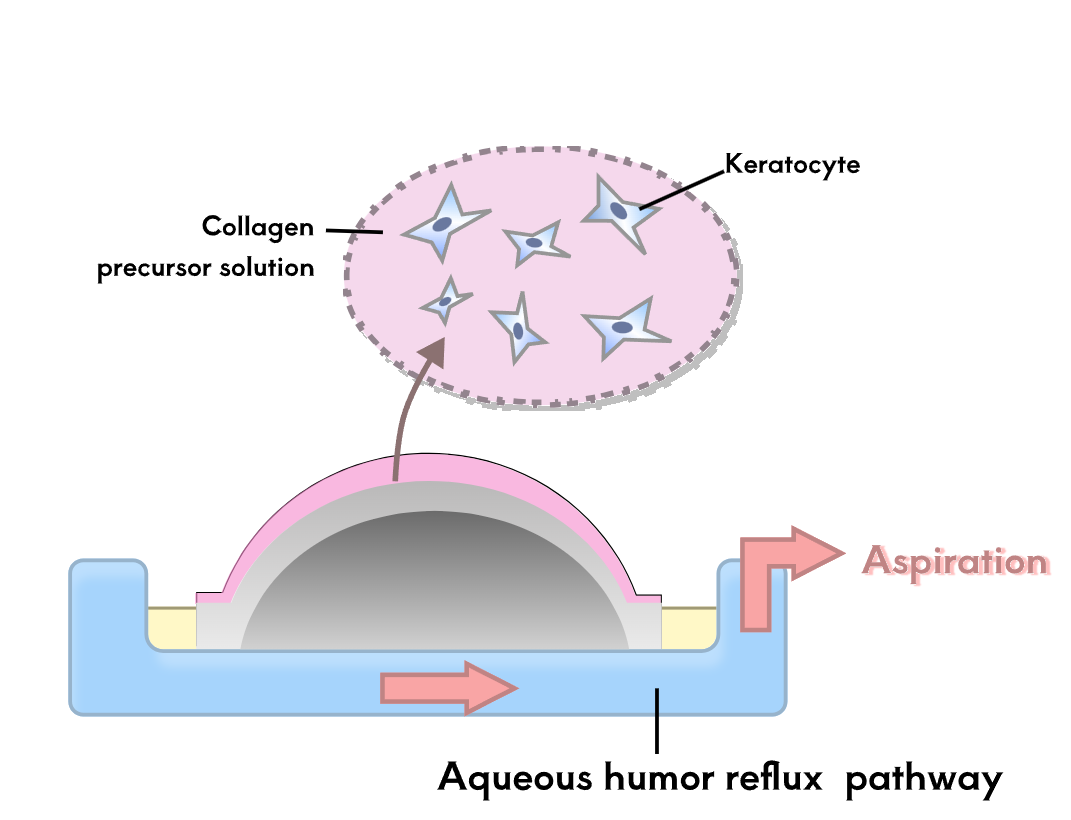
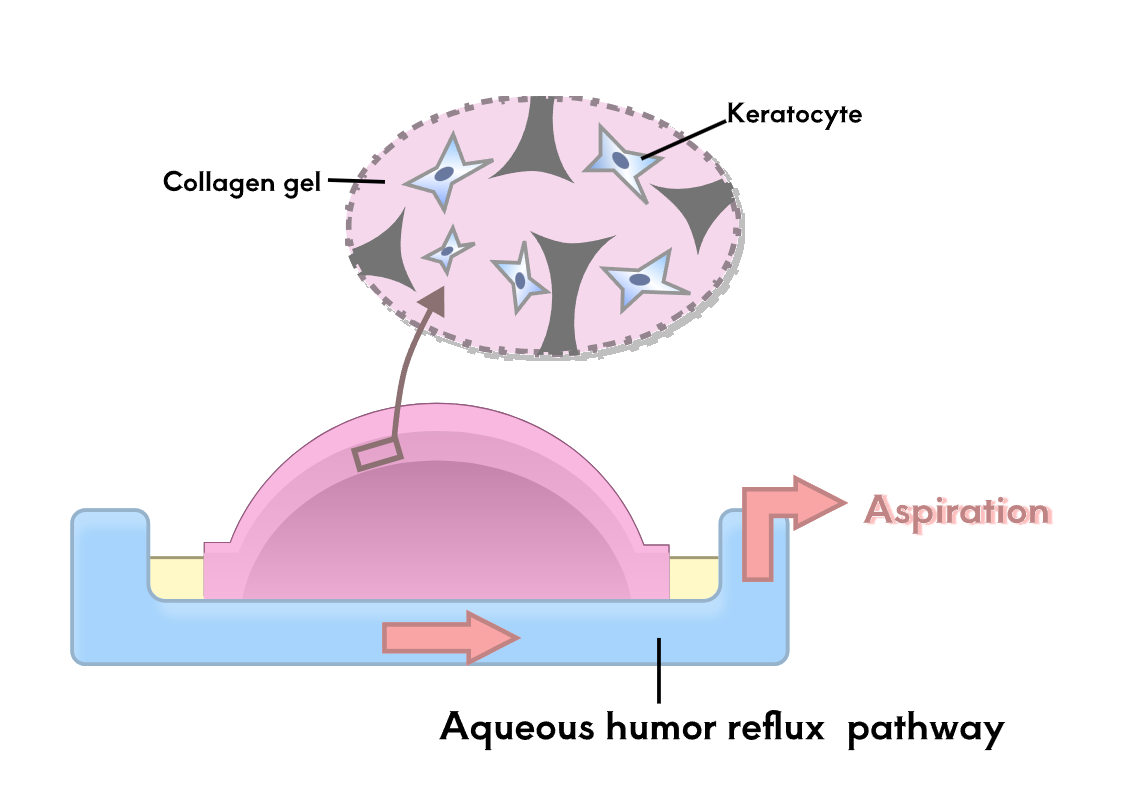
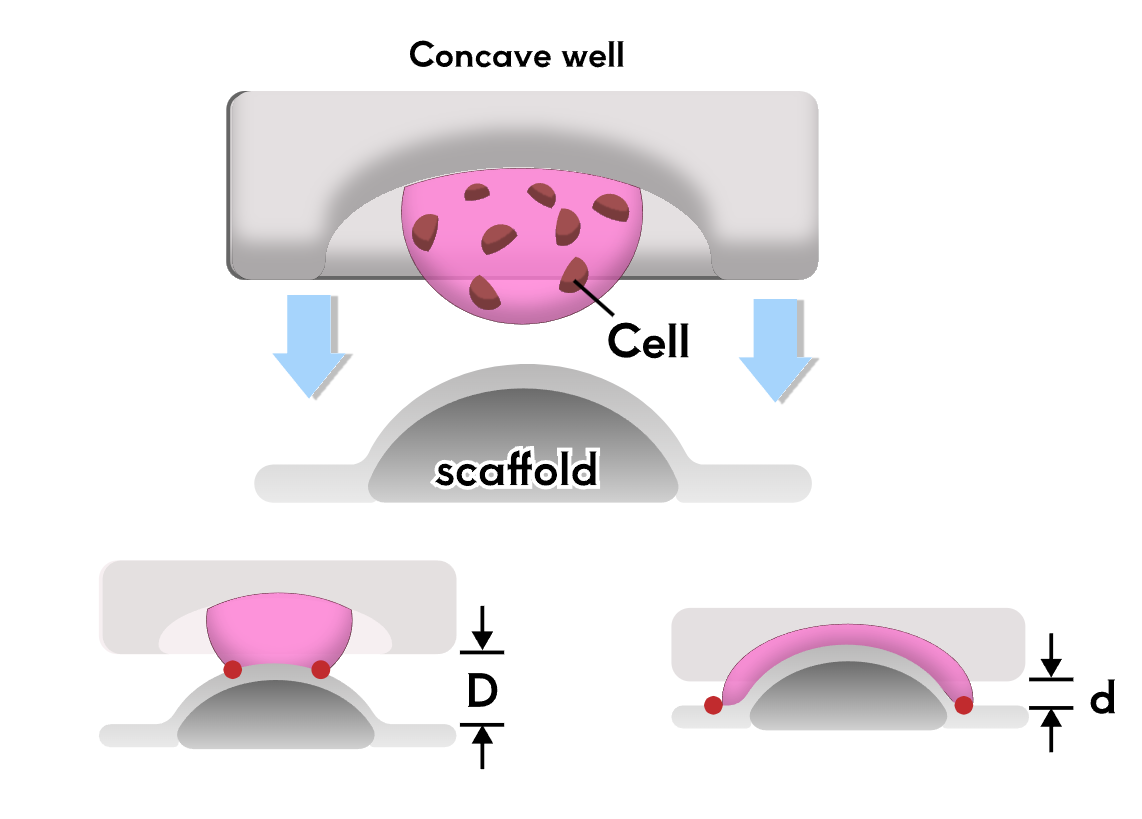
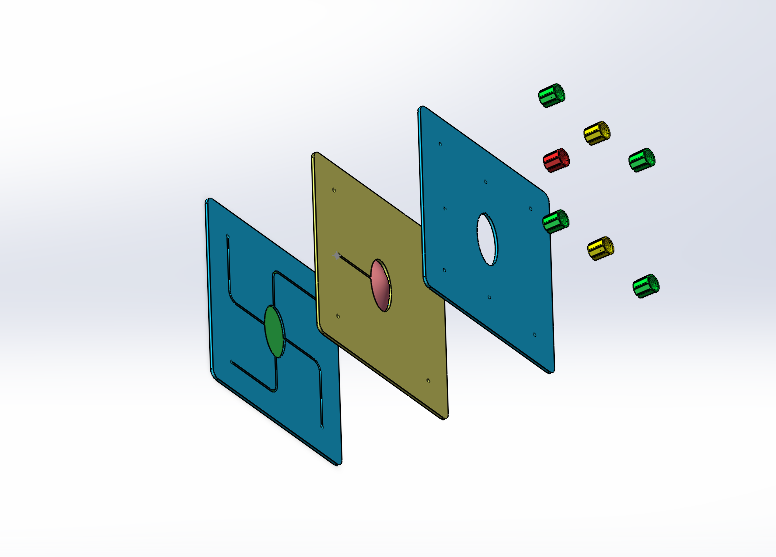
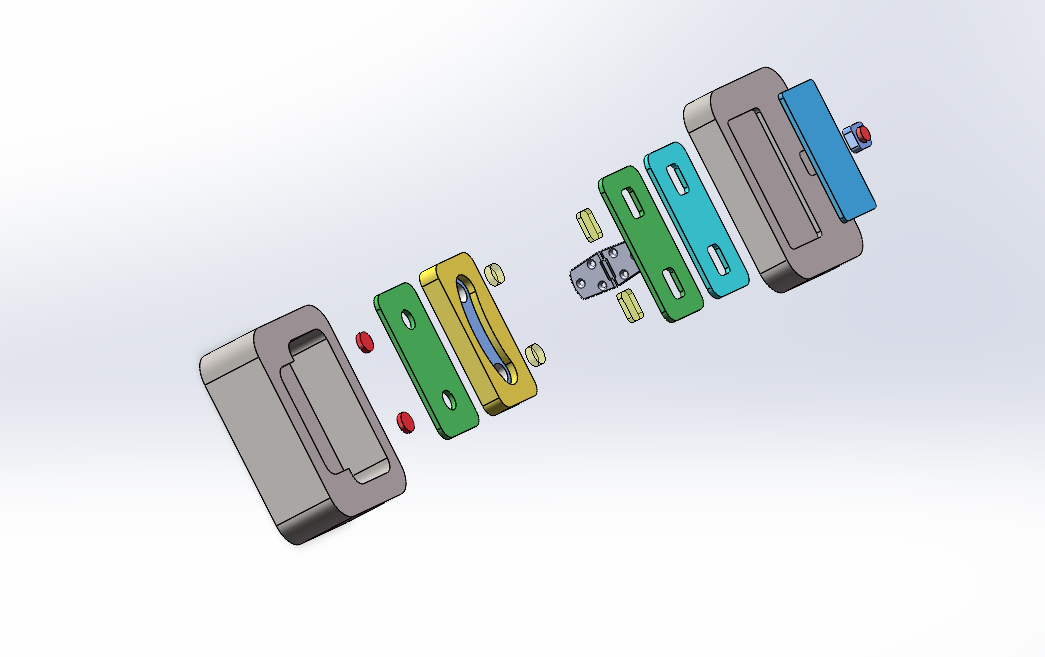
To test the therapeutic effect of the developed contact lenses, it is necessary to conduct biological experiments to verify the sustained release of RNF114 and the final amount of drug delivered to the lens. Since animal experiments are not within the scope of iGEM, we utilize microfluidic technology by stacking multilayer chips to simulate the drug delivery path in the human eye. This approach enables convenient, fast, and reproducible experiments, while also providing an iterative solution for the evaluation of ocular drug delivery.
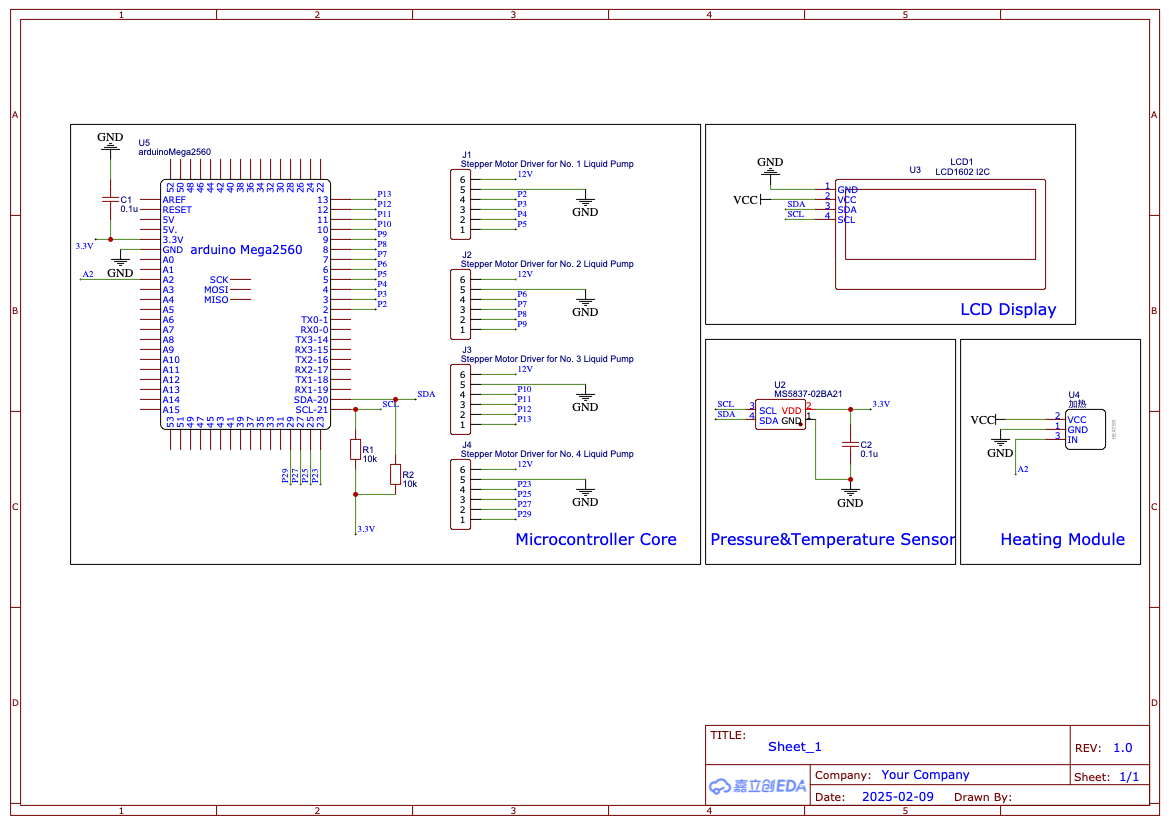
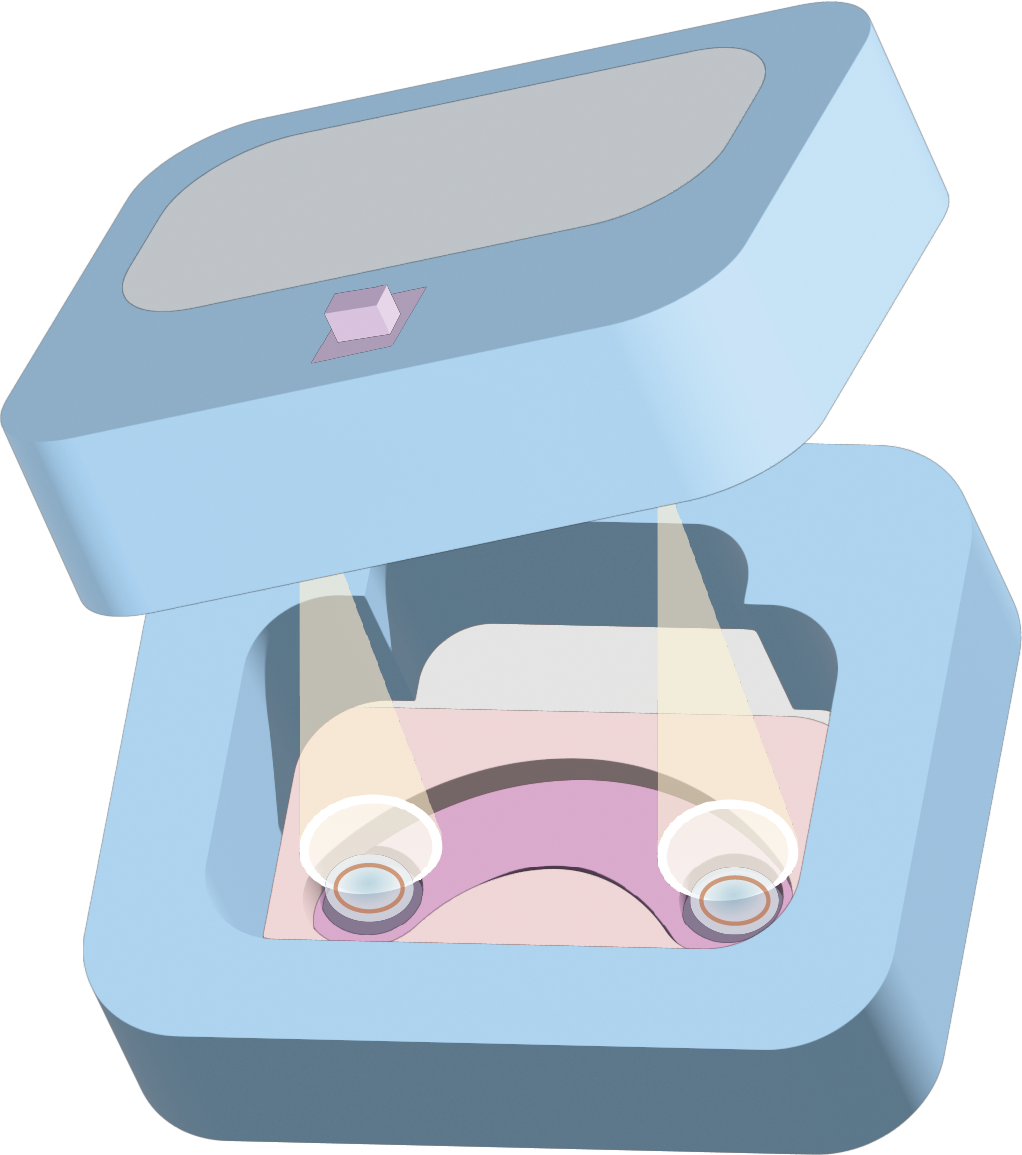
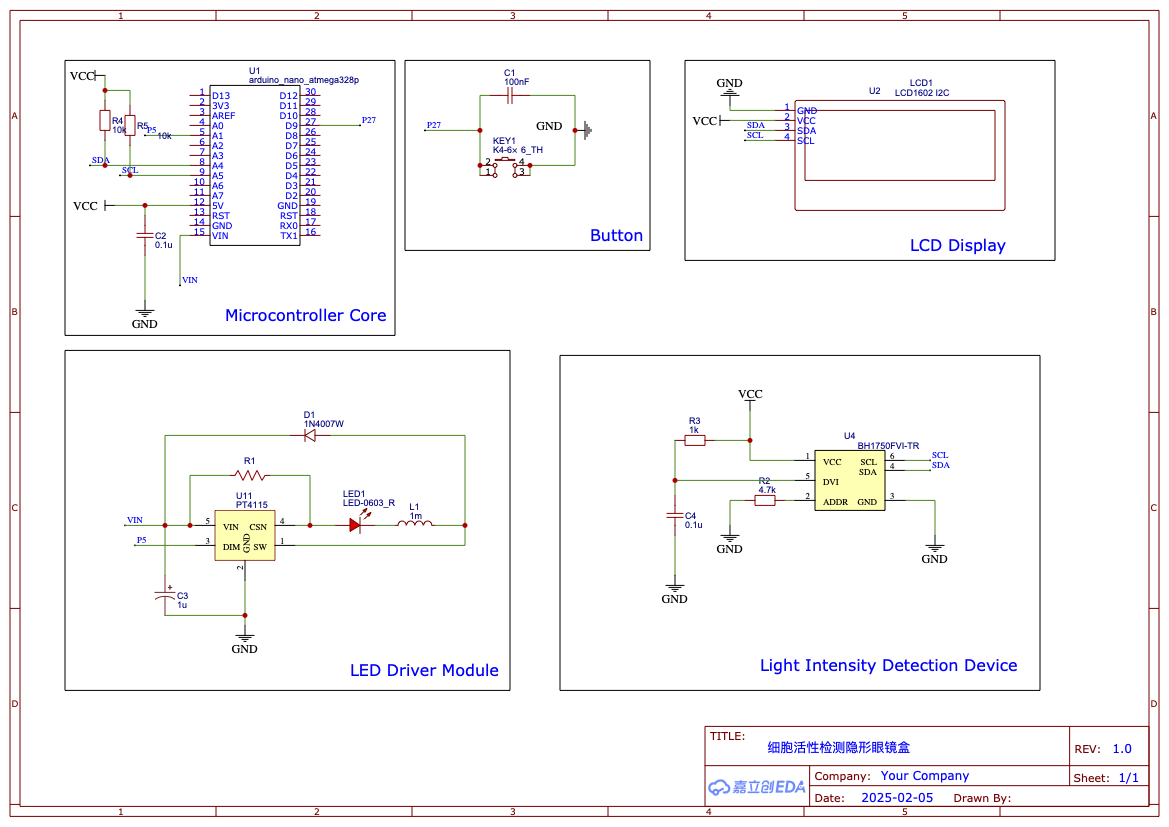
At the same time, to alert users to replace the contact lenses in time when the microbial activity declines, ensuring that the microbes remain highly active and continue to effectively secrete RNF114 while avoiding contamination of the eye from the microbial lysate, we have designed a user-friendly contact lens case. This case integrates a fluorescence detection device, which detects the active fluorescent signal emitted by the embedded bioreactors in the contact lenses. This helps users quickly determine whether the contact lenses are still active and safe for continued use.
Here are the two devices we designed:
- Eye Simulation Chip
- Cell Activity Detection Contact Lens Case